お問い合わせ
-
各種お問い合わせはこちらから www.quantiferon.com/jp/contact-us/
QuantiFERON-TB Gold (QFT) is a simple blood test that aids in the detection of Mycobacterium tuberculosis, the bacteria which causes tuberculosis (TB). QFT is an interferon-gamma (IFN-γ) release assay, commonly known as an IGRA, and is a modern alternative to the tuberculin skin test (TST, PPD or Mantoux). Unlike the TST, QFT is a controlled laboratory test that requires only one patient visit and is unaffected by previous Bacille Calmette-Guerin (BCG) vaccination.
QFT is highly specific and sensitive: a positive result is strongly predictive of true infection with M. tuberculosis. However, like the TST and other IGRAs, QFT cannot distinguish between active tuberculosis disease and latent tuberculosis infection, and is intended for use with risk assessment, radiography, and other medical and diagnostic evaluations. Like any diagnostic aid, QFT cannot replace clinical judgment.
The US Centers for Disease Control (CDC) Guidelines recommend the use of IGRAs in all situations in which the TST was historically used, with IGRAs being the preferred test for persons who have been BCG vaccinated or are unlikely to return for TST reading (1).
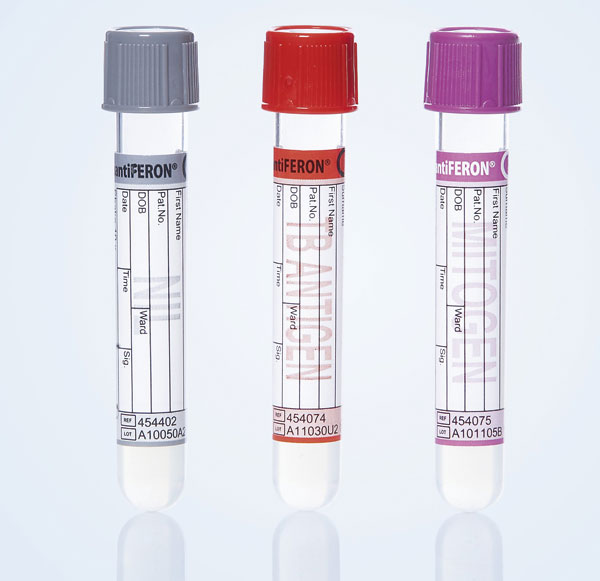
QFT uses unique blood collection tubes that enable immediate exposure of viable blood lymphocytes to highly specific TB antigens (ESAT-6/CFP-10/TB-7.7(p4)) and test controls coated on the inner surface of the tubes. Antigen exposure produces a quantifiable immune response to aid in the diagnosis of TB infection.
Negative control to adjust for background IFN-γ
To detect the CD4+ T cell responses to TB antigens
Low response may indicate inability to generate IFN-γ
QuantiFERON-TB Gold is the most clinically tested and proven IGRA available (2). More than 30 million QFT tests have been sold across 130+ countries, including more than 7 million in 2015. Trust the only blood test for TB infection that offers:
QFT employs whole blood collection to make T cell incubation simple and fast. After blood collection, the sample must be incubated, which can occur on-site or at the testing laboratory, providing your practice with complete flexibility and convenience.

Product claims may differ from country to country based on regulations and approvals. Contact your country representative for further details.
各種お問い合わせはこちらから www.quantiferon.com/jp/contact-us/