お問い合わせ
-
各種お問い合わせはこちらから www.quantiferon.com/jp/contact-us/

The advantages of QuantiFERON technology include the use of undiluted whole blood and the detection and measurement of IFN-γ in undiluted plasma samples. QuantiFERON assays are accurate and sensitive in whole blood and undiluted plasma (1).
The use of whole blood in QuantiFERON methods makes T cell incubation simple and fast. There is no tedious lymphocyte isolation, washing, counting, diluting, or culturing. Just shake each tube and incubate 16–24 hours. The simple blood incubation allows processing of a very large number of samples. Logistics are very simple and the QuantiFERON method allows virtually labor-free incubation at nearly every location. QuantiFERON technology preserves the in vivo cellular and biochemical environment for lymphocyte stimulation, maximizing cellular responses. The technology is designed for clinical screening of large sample numbers, providing the standards, controls, and assay reproducibility needed for clinical diagnosis (1).
Individuals exposed to disease/infection have specific T cell lymphocytes in their blood that maintain an immunological memory for the antigens (immunologically reactive molecules) of the priming disease/infection (2–4). The addition of antigen to blood collected from a primed individual results in the rapid restimulation of antigen-specific effector T cells, resulting in the release of cytokines (e.g., IFN-γ). Effector T cells are able to respond quickly when exposed to the priming antigen. Thus the production of IFN-γ in response to antigen exposure is a specific marker for cellular immune response against that antigen (recall response). This IFN-γ response may be used to quantify the recall response (5).
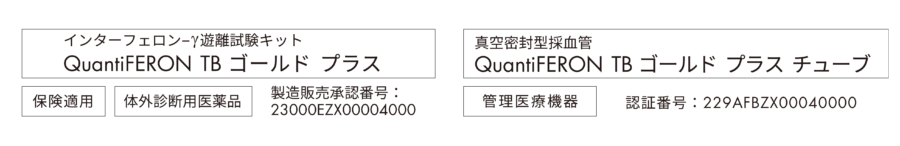
Product claims may differ from country to country based on regulations and approvals. Contact your country representative for further details.
各種お問い合わせはこちらから www.quantiferon.com/jp/contact-us/